Max Planck
Biography of M. K. E. L. Planck
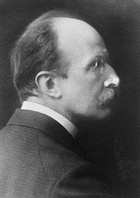 Max Karl Ernst Ludwig
Planck was born in Kiel, Germany, on April 23,
1858, the son of Julius Wilhelm and Emma (née Patzig) Planck.
He was Privatdozent in Munich from 1880 to 1885, then Associate
Professor of Theoretical Physics at Kiel until 1889, in which year
he succeeded Kirchhoff as Professor at Berlin University, where
he remained until his retirement in 1926. Afterwards he became President
of the Kaiser Wilhelm Society for the Promotion of Science, a post
he held until 1937. Max Karl Ernst Ludwig
Planck was born in Kiel, Germany, on April 23,
1858, the son of Julius Wilhelm and Emma (née Patzig) Planck.
He was Privatdozent in Munich from 1880 to 1885, then Associate
Professor of Theoretical Physics at Kiel until 1889, in which year
he succeeded Kirchhoff as Professor at Berlin University, where
he remained until his retirement in 1926. Afterwards he became President
of the Kaiser Wilhelm Society for the Promotion of Science, a post
he held until 1937.
Planck's earliest work was on the subject of
thermodynamics, an interest he acquired from his
studies under Kirchhoff, whom he greatly admired,
and very considerably from reading R. Clausius'
publications. He published papers on entropy, on
thermoelectric ity and on the theory of dilute
solutions.
At the same time also the problems of radiation
processes engaged his attention and he showed
that these were to be considered as
electromagnetic in nature. From these studies he
was led to the problem of the distribution of
energy in the spectrum of full radiation.
Experimental observations on the wavelength
distribution of the energy emitted by a black
body as a function of temperature were at
variance with the predictions of classical
physics. Planck was able to deduce the
relationship between the ener gy and the
frequency of radiation. In a paper published in
1900, he announced his derivation of the
relationship: this was based on the revolutionary
idea that the energy emitted by a resonator could
only take on discrete values or quanta. The
energy for a resonator of frequency v is hv
where h is a universal constant, now
called Planck's constant.
Planck faced a troubled and tragic period in his
life during the period of the Nazi government in
Germany, when he felt it his duty to remain in
his country but was openly opposed to some of the
Government's policies, particularly as regards
the persecuti on of the Jews. In the last weeks
of the war he suffered great hardship after his
home was destroyed by bombing. He died at
Göttingen on October 3, 1947.
http://www.nobel.se/laureates/physics-1918-1-bio.html
Max Karl Ernst Ludwig Planck Winner of
the 1918 Nobel Prize in Physics
1918 Nobel Laureate in Physics
"in recognition of the services he rendered
to the advancement of Physics by his discovery of
energy quanta."
http://www.nobelprizes.com/nobel/physics/1918a.html
 Max Planck - Nobel Prize Physics 1918 Max Planck - Nobel Prize Physics 1918
"in recognition of the
services he rendered to the advancement of
Physics by his discovery of energy quanta"
http://www.nobel.se/laureates/physics-1918.html
Max Planck
Max Planck made many contributions
to theoretical physics, but his fame rests
primarily on his role as originator of the
quantum theory. This theory revolutionized our
understanding of atomic and subatomic processes,
just as Albert Einstein's theory of relativity
revolutionized our understanding of space and
time. Together they constitute the fundamental
theories of 20th-century physics. Both have
forced man to revise some of his most cherished
philosophical beliefs, and both have led to
industrial and military applications that affect
every aspect of modern life.
Indeed, it was years before the
far-reaching consequences of Planck's achievement
were generally recognized, and in this Einstein
played a central role. In 1905, independently of
Planck's work, Einstein argued that under certain
circumstances radiant energy itself seemed to
consist of quanta (light quanta, later called
photons), and in 1907 he showed the generality of
the quantum hypothesis by using it to interpret
the temperature dependence of the specific heats
of solids. In 1909 he introduced the
wave-particle duality into physics.
Ironically, Planck himself was one of the last to
struggle for a return to classical theory, a
stance he later regarded not with regret but as a
means by which he had thoroughly convinced
himself of the necessity of the quantum theory.
Opposition to Einstein's radical light quantum
hypothesis of 1905 persisted until after the
discovery of the Compton effect in 1922.
http://astro.estec.esa.nl/SA-general/Projects/Planck/mplanck/mplanck.html
 Max Goes Online - Max Planck Max Goes Online - Max Planck
Bei Lichte betrachtet
müssen wir mit Fug und Recht als erstes Wunder
die Tatsache verzeichnen, daß wir überhaupt in
der Natur Gesetzmäßigkeiten vorfinden, die für
die Menschen aller Länder, Völker und Rassen
genau die gleichen sind. Sinn und
Grenzen der exakten Wissenschaft, Vortrag
gehalten im November 1942
http://www.karlsruhe.de/Schulen/Max-Planck-Gymnasium/planck/max.html
Max Planck
Max Planck was a German physicist
who lived between 1858-1947. His theories changed
our understanding of atomic processes and started
the field of quantum physics, which studies
energy inside atoms. Many of Planck's ideas were
later used by Einstein when he
developed his theory of relativity.
http://windows.ivv.nasa.gov/cgi-bin/tour_def/people/modern_era/planck.html
PLANCK, Max
(1858-1947). Awarded the Nobel prize
for physics in 1918, German physicist Max Planck
is best remembered as the originator of the
quantum theory (see Quantum Mechanics ). His work
helped usher in a new era in theoretical physics
and revolutionized the scientific community's
understanding of atomic and subatomic processes.
http://www.optonline.com/comptons/ceo/03769_A.html
 Max Planck Max Planck
The German physicist Max Karl Ernst
Ludwig Planck, b. Apr. 23, 1858, d. Oct. 3, 1947,
developed the concept of the quantum, or
fundamental increment of energy--basic to quantum
mechanics, and a cornerstone of modern physics.
After receiving his Ph.D. from the University of
Munich in 1879, Planck taught at the University
of Kiel (1885-89) and the University of Berlin
(1889-1926). His appointment at the latter
institution included the directorship of the
Institute of Theoretical Physics that was newly
founded for him.
http://sirius.phy.hr/~dpaar/fizicari/xplanck.html
Planck
By combining the formulas of Wien and Rayleigh, Planck
announced in 1900 a formula now known as Planck's
radiation formula. In a letter written a year
later Planck described proposing the formula
saying:-
...
the whole procedure was an act of despair because
a theoretical interpretation had to be found at
any price, no matter how high that might be.
Within
two months Planck made a complete theoretical
deduction of his formula renouncing classical
physics and introducing the quanta of energy. At
first the theory met resistance but due to the
successful work of Niels Bohr in 1913,
calculating positions of spectral lines using the
theory, it became generally accepted. Planck
himself explains how despite having invented
quantum theory he did not understand it himself
at first:-
I tried immediately
to weld the elementary quantum of action
somehow in the framework of classical theory.
But in the face of all such attempts this
constant showed itself to be obdurate ... My
futile attempts to put the elementary quantum
of action into the classical theory continued
for a number of years and they cost me a
great deal of effort.
Planck
received the Nobel Prize for Physics in 1918.
http://www-groups.dcs.st-and.ac.uk/~history/Mathematicians/Planck.html
Max Planck
Max Karl Ernst Ludwig Planck was
born on 23 April in Kiel Germany. In 1874 Planck
entered the University of Munich. Planck was
deeply impressed by the law of conservation of
energy and he became convinced that the second
law of termodynamics was an absolute law of
nature. Planck based his doctoral dissertation on
the second law of thermodynamics and in July
1879, at the young age of 21 he received his
doctoral degree.
Planck was intrigued by the law
discovered by his colleague Wilhelm Wien in 1896.
He made several attempts at deriving this law,
starting from the second law of thermodynamics as
a base Experimental evidence was coming to light
which showed that Wiens law broke down completely
at low frequencies but was perfectly viable at
high frequencies. Plank guessed that, since the
entropy of radiation depended mathematically on
it's energy in the high frequency range due to
Wiens law, and that because he knew what the
dependance was in the low frequency region, he
should somehow combine these two properties in
some simple manner resulting in a formula
relating frequency, to the energy of radiation.
The formula was hailed a great success, but
Planck noted that it was just a formula; a lucky
guess which still had to be derived from first
principles in order to give it a proper
scientific standing.
In 1900, at the age of 42, Planck
achieved this, but in the process he had to
abandon one of his greatest beliefs - that the
second law of thermodynamics was an absolute law
of nature. He was forced to accept Ludwig
Boltzmann's statistical explanation for the
second law. Planck also had to assume that the
black body oscillators could only absorb and emit
energy in discrete amounts of energy -
packets of energy which he called quanta.
http://www.physics.gla.ac.uk/introPhy/Famous/planck/planck.html
 Planck Planck
Born: 23 April 1858 in Kiel, Schleswig-Holstein,
Germany
Died: 4 Oct 1947 in Göttingen, Germany
Max Planck initiated the study of quantum
mechanics when he announced in 1900 his
theoretical research into radiation and
absorption of a black body.
Planck describes why he chose
physics:-
The outside
world is something independent from man,
something absolute, and the quest for the
laws which apply to this absolute appeared to
me as the most sublime scientific pursuit in
life.
http://www.vma.bme.hu/mathhist/Mathematicians/Planck.html
Planck, Max (1858-1947)
German physicist who formulated an equation describing
the Blackbody spectrum in 1900. Wien and Rayleigh had also developed equations,
but Wien's only worked at high frequencies,
and Rayleigh's only worked at low frequencies.
Planck's spectrum was obtained by postulating that energy was directly
proportional to frequency (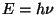 ). Planck believed that this quantization applied
only to the the absorption and emission of energy by matter, not
to electromagnetic waves themselves. However, it turned out to be
much more general than he could have imagined. ). Planck believed that this quantization applied
only to the the absorption and emission of energy by matter, not
to electromagnetic waves themselves. However, it turned out to be
much more general than he could have imagined.
http://www.treasure-troves.com/bios/Planck.html
About Max Planck
Max Karl Ernst Ludwig Planck was
born on April 23, 1858 in Kiel, Schleswig-Holstein
(Germany), as the sixth child of a professor of
law at the university of Kiel.
While in Berlin Planck studied thermodynamics, in
particular examining the distribution of energy
according to wavelength. By combining the
formulas of Wien and Rayleigh, Planck announced
in 1900 a formula now known as Planck's
»radiation formula« or »Planck's
constant h«.
Within two months he made a complete theoretical
deduction of his formula renouncing classical
physics and introducing the »quanta of energy«.
At first his theory found strong resistance but
due to the successful work of Niels Bohr in 1913,
calculating positions of spectral lines using
Planck's theory, it became generally accepted.
At
the end of World War II, American officers took
Planck and his wife to Göttingen, where he died
on October 4, 1947, in his 89th year.
http://www.mpg.es.bw.schule.de/allgemein/maxplanck/max_plan.htm
Max Planck
The German physicist Max Karl Ernst
Ludwig Planck, b. Apr. 23, 1858, d. Oct. 3, 1947,
developed the concept of the quantum, or
fundamental increment of energy, basic to quantum
mechanics, and a cornerstone of modern physics.
http://www.electro-optical.com/bb_rad/mplanck.htm
Max Planck
German physicist and Nobel laureate,
who was the originator of the quantum theory.
Planck was born in Kiel on April 23, 1858, and
educated at the universities of Munich and
Berlin. In 1900 Planck postulated that energy is
radiated in small, discrete units, which he
called quanta. Developing his quantum theory
further, he discovered a universal constant of
nature, which came to be known as Planck's
constant. Planck's law states that the energy of
each quantum is equal to the frequency of the
radiation multiplied by the universal constant.
Planck received many honors for his work, notably
the 1918 Nobel Prize in physics.
http://www.demogr.mpg.de/General/Member/MaxP.htm
Max Planck, "Revolutionär wider Willen"
Jürgen Langenbach - DER STANDARD, 13. Dezember 2000
Vor hundert Jahren wurden die Grundlagen der Quantenphysik gelegt
Vor hundert Jahren begann die seltsamste aller Revolutionen. Sie prägt zwar unser Alltagsleben rundum - kein Computer liefe ohne sie -, ist aber unserem Alltagsbewusstsein fremd geblieben. Und ihr Betreiber war kein hitzköpfiger Weltverbesserer, sondern ein "absolut unrevolutionärer Protoyp des preußischen Geheimrats", erklärt Anton Zeilinger (Experimentalphysik, Uni Wien) dem STANDARD: Max Planck, der "Revolutionär wider Willen".
http://derstandard.at/dyn/archiv/archarchiv.asp?artfn=/archiv/20001213/101.htm&strTitle=Max+Planck%2C+%22Revolution%E4r+wider+Willen%22
PLANCK'S CONSTANT
Fundamental physical constant,
symbol h, first discovered (1900) by the German
physicist Max Planck. Until that year, light in
all forms had been thought to consist of waves.
http://www.fwkc.com/encyclopedia/low/articles/p/p020000016f.html
Planck's Constant and the Energy of a
Photon
In 1900, Max Planck was working on
the problem of how the radiation an object emits
is related to its temperature. He came up with a
formula that agreed very closely with
experimental data, but the formula only made
sense if he assumed that the energy of a
vibrating molecule was quantized--that is,
it could only take on certain values. The energy
would have to be proportional to the frequency of
vibration, and it seemed to come in little
"chunks" of the frequency multiplied by
a certain constant. This constant came to be
known as Planck's constant, or h,
and it has the value
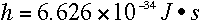
http://www.colorado.edu/physics/2000/quantumzone/photoelectric2.html
Biography of Max Planck
Raul Barron
Max Planck made many contributions
to theoretical physics and attained his fame via
his role as originator of the quantum theory.
This theory revolutionized our understanding of
atomic and subatomic processes, it is one of the
fundamental theories of 20th-century physics, and
has led to industrial and military applications
that affect every aspect of modern life.
http://wwwchem.csustan.edu/chem3070/Raul1.htm
ESVA: Planck Mini-Exhibit
 "It is impossible
to make a clear cut between science, religion, and art. The whole
is never equal simply to the sum of its various parts." --Max
Planck "It is impossible
to make a clear cut between science, religion, and art. The whole
is never equal simply to the sum of its various parts." --Max
Planck
In
his Scientific Autobiography, Max Planck
penned a now familiar adage known as Planck's
Principle:
"A
new scientific truth does not triumph by
convincing its opponents and making them see the
light, but rather because its opponents
eventually die, and a new generation grows up
that is familiar with it."
http://www.aip.org/history/esva/exhibits/planck.htm
A Science Odyssey: People and
Discoveries: Planck discovers the quantum nature
of energy
In 1899 Max Planck became a
professor at the University of Berlin, after nine
years at the University of Munich and Kiel
University, in Germany. In his research there, he
turned to a thermodynamic problem raised by one
of his old teachers. The problem was that of a
"black body," something that absorbs
all frequencies (or wavelengths) of light. When
heated it should then radiate all frequencies of
light equally -- theoretically. But the
distribution of energy radiated in "real
life" never matched up with the predictions
of classical physics.
Planck was as steeped in traditional physics as
his colleagues, but he had an open mind. The
older way wasn't working. So he changed one basic
assumption: energy, instead of being continuous,
comes in distinct particles. These were later
called "quanta," from the Latin for
"how much?" Though it sounded
outlandish, applying this idea to the problem of
heated bodies revealed a simple relationship that
explained previous puzzles. Planck found that the
energy radiated from a heated body is exactly
proportional to the wavelength of its radiation.
So, a black body would not radiate all
frequencies equally. As temperature goes up,
energy increases and it's more likely that quanta
with higher energy will be radiated. So, as an
object heats up, the light given off is orange,
then yellow, and eventually bluish. The
wavelength emitted is a function of the energy
times a constant (h), now known as Planck's
constant.
In 1905, Albert Einstein used the
theory of quanta to accurately describe the
photoelectric effect. In 1913, Niels Bohr incorporated
Planck's idea into his revision of model of the atom, resolving
inconsistencies that classical physics could not.
http://www.pbs.org/wgbh/aso/databank/entries/dp00qu.html
quantum theory
In physics, the theory that energy does not
have a continuous range of values, but is,
instead, absorbed or radiated discontinuously, in
multiples of definite, indivisible units called
quanta. Just as earlier theory showed how light,
generally seen as a wave motion, could also in
some ways be seen as composed of discrete
particles (photons), quantum
theory shows how atomic particles such as
electrons may also be seen as having wavelike
properties. Quantum theory is the basis of
particle physics, modern theoretical chemistry,
and the solid-state physics that describes the
behaviour of the silicon chips used in computers.
The theory began with the work of Max Planck in
1900 on radiated energy, and was extended by
Albert Einstein to electromagnetic radiation
generally, including light. Danish physicist
Niels Bohr used it to explain the spectrum of light
emitted by excited hydrogen atoms. Later work by
Erwin Schrödinger, Werner Heisenberg, Paul
Dirac, and others elaborated the theory to what
is called quantum mechanics (or wave mechanics).
http://ukdb.web.aol.com/hutchinson/encyclopedia/18/M0029018.htm
Encyclopædia Britannica: Max Planck
Planck's concept of energy quanta
conflicted fundamentally with all past physical
theory. He was driven to introduce it strictly by
the force of his logic; he was, as one historian
put it, a reluctant revolutionary. Indeed, it was
years before the far-reaching consequences of
Planck's achievement were generally recognized,
and in this Einstein played a central role. In
1905, independently of Planck's work, Einstein argued that
under certain circumstances radiant energy itself
seemed to consist of quanta (light quanta, later
called photons), and in 1907 he showed the
generality of the quantum hypothesis by using it
to interpret the temperature dependence of the
specific heats of solids. In 1909 Einstein
introduced the wave-particle duality into
physics. In October 1911 he was among the group
of prominent physicists who attended the first
Solvay conference in Brussels. The discussions
there stimulated Henri Poincaré to provide
a mathematical proof that Planck's radiation law
necessarily required the introduction of
quanta--a proof that converted James (later Sir
James) Jeans and others into supporters of the quantum theory. In 1913 Niels Bohr also
contributed greatly to its establishment through
his quantum theory of the hydrogen atom.
Ironically, Planck himself was one of the last to
struggle for a return to classical theory, a
stance he later regarded not with regret but as a
means by which he had thoroughly convinced
himself of the necessity of the quantum theory.
Opposition to Einstein's radical light quantum
hypothesis of 1905 persisted until after the
discovery of the Compton effect in 1922.
http://britannica.com/bcom/eb/article/5/0,5716,115045+2,00.html
Max Planck
Max Planck, allemand,
est né le 23 avril 1858, à Kiel capitale du
Schleswif-Holsteil. Il est le sixième enfant
d'une vieille famille de la haute bourgoisie.
Pour expliquer la loi qui donne la valeur
"E" de l'énergie rayonnée par un
corps parfaitement absorbant (corps noir) en
fonction de la température absolue "T"
de ce corps, et de la longueur d'onde (lamda) de
la radiation émise, Planck introduit cette idée
révolutionnaire dans la physique de son temps,
à savoir que l'énergie rayonnée par un corps
noir est un multiple entier du nombre hf,
"f" étant la fréquence émise et
"h" la constante de Planck. Avec cette
hypothèse, l'énergie "E"
correspondant à une température "T"
et à une longueur d'onde est donnée par la
formule E=hf (E=hc/lamda).
Dans cette formule, "h" est la
constante de Planck et "c" est la
vistesse de la lumière (c=3 X108
m/s). Elles sont des constantes bien connues.
http://mendeleiev.cyberscol.qc.ca/chimisterie/9606/CNadeau.html
Quotations by Planck
"If anybody says he can
think about quantum problems without getting
giddy, that only shows he has not understood the
first thing about them."
http://turnbull.dcs.st-and.ac.uk/history/Quotations/Planck.html
|



