Ernest
Rutherford
Ernest Rutherford
 Ernest
Rutherford 1st Baron Rutherford of Nelson
and Cambridge (1871-1937), British
physicist, who became a Nobel laureate for his
pioneering work in nuclear physics and for his
theory of the structure of the atom. Rutherford
was born on August 30, 1871, in Nelson, New
Zealand, and was educated at the University of
New Zealand and the University of Cambridge.
Rutherford was one of the first and most
important researchers in nuclear physics. Soon
after the discovery of radioactivity in 1896 by
the French physicist Antoine Henri Becquerel,
Rutherford identified the three main components
of radiation and named them alpha, beta, and
gamma rays. He also showed that alpha particles
are helium nuclei. His study of radiation led to
his formulation of a theory of atomic structure,
which was the first to describe the atom as a
dense nucleus which electrons circle. In 1919
Rutherford conducted an important experiment in
nuclear physics when he bombarded nitrogen gas
with alpha particles and obtained atoms of an
isotope of oxygen and protons. This transmutation
of nitrogen into oxygen was the first
artificially induced nuclear reaction. He was
awarded the 1908 Nobel Prize for Chemistry, was
knighted in 1914, and was made a baron in 1931.
He died in London on October 19, 1937, and was
buried in Westminster Abbey. Ernest
Rutherford 1st Baron Rutherford of Nelson
and Cambridge (1871-1937), British
physicist, who became a Nobel laureate for his
pioneering work in nuclear physics and for his
theory of the structure of the atom. Rutherford
was born on August 30, 1871, in Nelson, New
Zealand, and was educated at the University of
New Zealand and the University of Cambridge.
Rutherford was one of the first and most
important researchers in nuclear physics. Soon
after the discovery of radioactivity in 1896 by
the French physicist Antoine Henri Becquerel,
Rutherford identified the three main components
of radiation and named them alpha, beta, and
gamma rays. He also showed that alpha particles
are helium nuclei. His study of radiation led to
his formulation of a theory of atomic structure,
which was the first to describe the atom as a
dense nucleus which electrons circle. In 1919
Rutherford conducted an important experiment in
nuclear physics when he bombarded nitrogen gas
with alpha particles and obtained atoms of an
isotope of oxygen and protons. This transmutation
of nitrogen into oxygen was the first
artificially induced nuclear reaction. He was
awarded the 1908 Nobel Prize for Chemistry, was
knighted in 1914, and was made a baron in 1931.
He died in London on October 19, 1937, and was
buried in Westminster Abbey.
http://library.thinkquest.org/13822/RUTHERFO/ruther.html
Nobelprize Chemistry 1908: Ernest
Rutherford
"for his investigations into
the disintegration of the elements, and the
chemistry of radioactive substances"
http://www.nobel.se/laureates/chemistry-1908.html
Ernest Rutherford
Ernest Rutherford was born at
Bridgewater, close to Nelson, New Zealand. His
parents had emigrated to New Zealand from Britain
approximately 30 years earlier. At the age of 28
Rutherford took up the position of professor at
the University of McGill in Montreal, Canada,
carrying out research into radioactivity. The
some of the most important work was in the
identification of the alpha, beta and gamma
radiation. In 1902, with the collaboration of
Frederick Soddy, he enunciated and verified the
'spontaneous transformation' theory of
radioactive decay, whereby a radioactive atom
changes to a different atom on the emission of
radiation.
Rutherford received the Noble prize in Chemistry
for his work in radioactivity in 1908. With the
help of 15 research students, Rutherford was
carrying out research on the nature of alpha
particles. This research led him to believe that
alpha particles were in fact Helium atoms. In
1908 Rutherford peformed a simple experiment
involving the radioactive gas radon which decays
by emitting alpha particles. The gas was placed
within a glass container which also contained an
evacuated glass tube containing electrodes at
each end (similar to a fluorescent tube). The
apparatus was left for a few days after which a
high voltage was applied across the two
electrodes, the resulting electric discharge was
analysed with the emission spectrum the same as
that produced by the electric discharge from
Helium gas. The alpha particles had travelled
through the glass walls of the evacuated tube to
collect as Helium gas.
In 1911 Rutherford announced his most
revolutionary idea on the nature of the atom.
Rutherford proposed that the atom was constructed
with a small densely packed positive nucleus with
orbiting electrons - the solar system model. In
1912 Niels Bohr joined with Rutherford and
published his theories on the nature of the atom.
http://www.physics.gla.ac.uk/introPhy/Famous/rutherford/rutherford.html
Ernest Rutherford (1871-1937)
Rutherford, Ernest (1871-1937): Born
in New Zealand, Rutherford studied under J. J.
Thomson at the Cavendish Laboratory in England.
His work constituted a notable landmark in the
history of atomic research as he developed Bacquerel's discovery
of Radioactivity into an exact and documented
proof that the atoms of the heavier elements,
which had been thought to be immutable, actually
disintegrate (decay) into various forms of
radiation.
http://www.chemistry.co.nz/ernest_rutherford.htm
ChemTeam: Rutherford atom abstract of
1911
Ernest Rutherford
The Scattering of the a and b Rays
and the Structure of the Atom, by
PROFESSOR E. RUTHERFORD, F.R.S. From the
Proceedings of the Manchester Literary and
Philosophical Society, IV, 55, pp. 18-20.
Abstract of a paper read before the Society on
March 7, 1911:
"It is well known that the a
and b particles are deflected from their
rectilinear path by encounters with the atoms of
matter. There seems to be no doubt that these
swiftly moving particles actually pass through
the atomic system, and a close study of the
deflexions produced should throw light on the
electrical structure of the atom. It has been
usually assumed that the scattering observed is
the result of a multitude of small scatterings.
There are, however, a number of experiments on
scattering, which indicate that an a or b
particle occasionally suffers a deflexion of more
than 90° in a single encounter. In order to
explain these and other results, it is necessary
to assume that the electrified particle passes
through an intense electric field within the
atom. The scattering of the electrified particles
is considered for a type of atom which consists
of a central electric charge concentrated at a
point and surrounded by a uniform spherical
distribution of opposite electricity equal in
amount."
http://dbhs.wvusd.k12.ca.us/Chem-History/Rutherford-atom-abstract.html
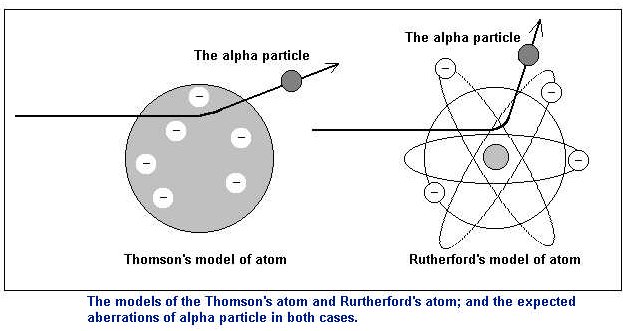
Rutherford's experiment
In the year 1898 Thomson created the
idea of atom as the positive charged ball in
which there are negative charges placed - the "plum cake"
model. So summing
up the whole atom were to be neutral.
In the years 1909-1911 Ernest Rutheford and his
students - Hans Geiger (1882-1945) and Ernest
Marsden conducted some experiments to search the
problem of alpha particles scattering by the thin
gold-leaf. Rutheford knew that the particles
contain the 2e charge. The experiment caused the
creation of the new model of atom - the
"planetary" model.
http://library.thinkquest.org/19662/low/eng/exp-rutherford.html
Sir Ernest Rutherford
Sir Ernest Rutherford, b. near
Nelson, New Zealand, Aug. 30, 1871, d. Oct. 19,
1937, perhaps more than any other scientist,
formed modern-day views concerning the nature of
matter. Rutherford's early work with Thomson led
to investigations of electricity and radiation
and eventually to a detailed study of
radioactivity. Although others had pioneered the
earliest developments in radioactivity,
Rutherford soon achieved dominance in this field.
He found that at least two kinds of radiation,
which he labeled alpha and beta, existed. Working
with Frederick Soddy in 1902-03, Rutherford
identified the phenomenon of radioactive
half-life and formulated the still-accepted
explanation of radioactivity: each decay of the
atoms of radioactive materials signifies the
transmutation of a parent element into a
daughter, with each type of atom having its own
transformation period. Rutherford was awarded the
1908 Nobel Prize for chemistry for his work in
radioactivity. Rutherford made his greatest
discovery in 1909. Shortly after his move to
Manchester, he found that a few alpha particles,
when bombarding thin metal foils, were deflected
from their incident beam through more than 90
deg. "It was almost as incredible,"
Rutherford later responded in a now-classic
statement, "as if you fired a fifteen-inch
shell at a piece of tissue paper and it came back
and hit you." Early in 1911 he finally
announced his version of the structure of the
atom: a very small, tightly packed, charged
nucleus sprinkled with opposite charges in the
mostly empty surrounding void.
http://faust.irb.hr/~dpaar/fizicari/xrutherf.html
Ernest Rutherford
 Ernest
Rutherford is considered the father of nuclear
physics. Indeed, it could be said that Rutherford
invented the very language to describe the
theoretical concepts of the atom and the
phenomenon of radioactivity. Particles named and
characterized by him include the alpha particle,
beta particle and proton. Even the neutron,
discovered by James Chadwick, owes its name to
Rutherford. For this work, Rutherford won the
1908 Nobel Prize in chemistry. In 1909, now at
the University of Manchester, Rutherford was
bombarding a thin gold foil with alpha particles
when he noticed that although almost all of them
went through the gold, one in eight thousand
would "bounce" (i.e. scatter) back. The
amazed Rutherford commented that it was "as
if you fired a 15-inch naval shell at a piece of
tissue paper and the shell came right back and
hit you." From this simple observation,
Rutherford concluded that the atom's mass must be
concentrated in a small positively-charged
nucleus while the electrons inhabit the farthest
reaches of the atom. Although this planetary
model of the atom has been greatly refined over
the years, it remains as valid today as when it
was originally formulated by Rutherford. Ernest
Rutherford is considered the father of nuclear
physics. Indeed, it could be said that Rutherford
invented the very language to describe the
theoretical concepts of the atom and the
phenomenon of radioactivity. Particles named and
characterized by him include the alpha particle,
beta particle and proton. Even the neutron,
discovered by James Chadwick, owes its name to
Rutherford. For this work, Rutherford won the
1908 Nobel Prize in chemistry. In 1909, now at
the University of Manchester, Rutherford was
bombarding a thin gold foil with alpha particles
when he noticed that although almost all of them
went through the gold, one in eight thousand
would "bounce" (i.e. scatter) back. The
amazed Rutherford commented that it was "as
if you fired a 15-inch naval shell at a piece of
tissue paper and the shell came right back and
hit you." From this simple observation,
Rutherford concluded that the atom's mass must be
concentrated in a small positively-charged
nucleus while the electrons inhabit the farthest
reaches of the atom. Although this planetary
model of the atom has been greatly refined over
the years, it remains as valid today as when it
was originally formulated by Rutherford.
http://www.orcbs.msu.edu/radiation/radhistory/ernestrutherford.html
 Ernest Rutherford Ernest Rutherford
Ernest Rutherford (1871-1937) was a
New Zealand-born English physicist known as the
"Father of Nuclear Physics." Rutherford
described that radiation was caused by the
destruction of atoms and that the alpha particle
is the helium nucleus. This was the basis of his
Nobel prize.
Rutherford also made two discoveries vital for
nuclearphysics - an atom consists of a small
nucleus surrounded by electrons, and by making
clear the basic structure of atoms, elements can
be artificially transmuted.
http://spaceboy.nasda.go.jp/note/Kagaku/E/kag113_rutherford_e.html
Rutherford Biography
Ernest Rutherford, British
physicist, who became a Nobel laureate for his
pioneering work in nuclear physics and for his
theory of the structure of the atom.
http://www2.slac.stanford.edu/vvc/nobel/rutherford.html
The Scientists: Ernest Rutherford
I was once again struck, in my
review of Rutherford's life, of how many great
persons in history have had simple beginnings.
Though he was to become one of the greatest
pioneers of subatomic physics, Ernest Rutherford
came from simple people, a family with
"heart, head, hand." He was born in
Spring Grove, South Island, New Zealand, the
fourth of twelve children. His father was a
"wheelwright and flaxmiller."
By 1910, Rutherford was beginning to
understand the nature of the inner structure of
the atom which led him to postulate the existence
within the atom of a concentrated part, the
"nucleus": this, indeed, was to be
Rutherford's greatest contribution to physics. During
1920, Rutherford was to predict the existence of
the neutron, which, a colleague of his, Sir James
Chadwick (1891-1974), was to in fact to discover
in 1932, and for which Chadwick was to receive a
Nobel in 1935.
http://www.blupete.com/Literature/Biographies/Science/Rutherford.htm
|



